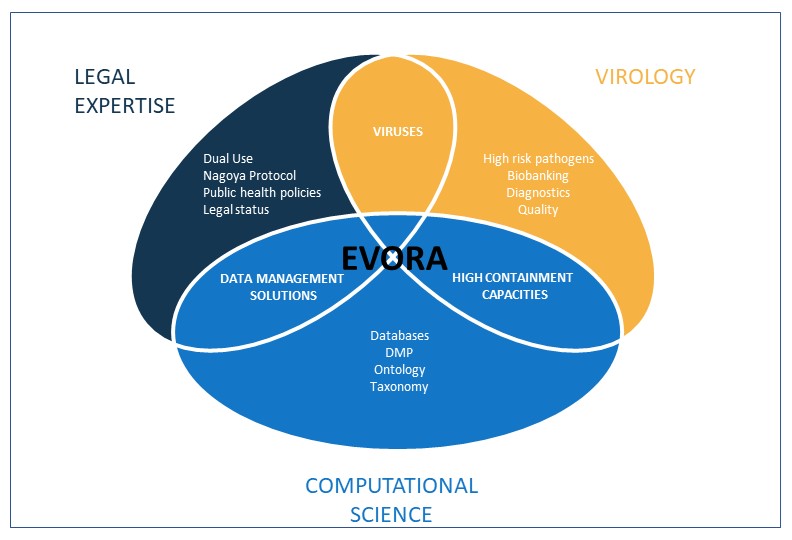
Our project brings together the expertise of EVA, ERINHA, and ELIXIR, uniting forces to create a robust framework for pandemic preparedness . . .
EVORA is building resilience for pandemics, addressing critical needs linked to viral emergence, mitigating collateral effects such as fragmented research, regulatory obstacles, and governance issues.
We are unifying our research infrastructure operations for optimal responsiveness, sustainability, and global competitiveness. This involves harmonizing procedures, creating a comprehensive online catalog, and implementing shared quality management guidelines.
EVORA endeavors to enhance the EU's capacity for pandemic preparedness research and response to viral diseases. This involves establishing a coherent and sustainable infrastructure framework that improves readiness at both fundamental and preclinical levels, strengthening the EU's capacity for coordinated research and action in the face of infectious disease threats.
EVORA aspires to offer a sustainable, operational alliance. This partnership is designed to provide tailored, integrated resources and preclinical research services, responding specifically to the study of viral pathogens with epidemic or pandemic potential.
EVORA recognizes the specific regulatory, ethical, and security challenges related to emerging pathogens. The project is committed to providing a solid framework for regulatory compliance, navigating complexities, and contributing to the evolution of policies and regulations.
The EVORA Project is an initiative at the forefront of pandemic preparedness and response. In a world facing escalating risks from emerging pathogens, including viruses with epidemic and pandemic potential, EVORA stands as a beacon of collaboration and innovation.
Our mission is clear: to build a comprehensive and coordinated framework that fortifies the European Union's capacity for concerted research and action in the face of infectious disease threats. EVORA aims to address the pressing needs associated with viral emergence, mitigating collateral effects such as fragmented research, regulatory obstacles, and governance issues.
EVORA aspires to offer a sustainable, operational alliance dedicated to providing customized, integrated resources and preclinical research services tailored to the study of viral pathogens with epidemic or pandemic potential.
Expected Results:
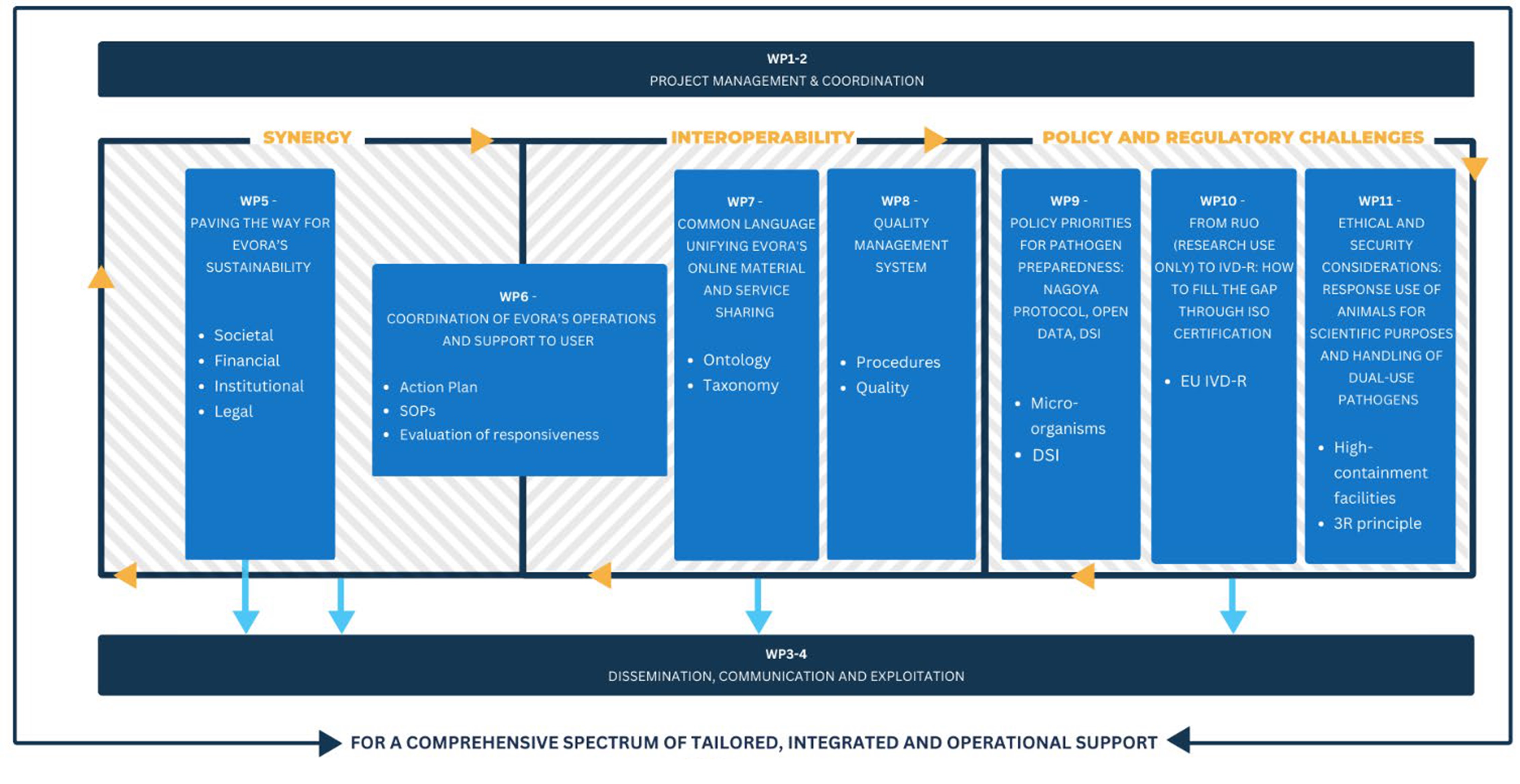
Each WP is meticulously designed to address specific aspects crucial for building resilience against viral threats. From establishing governance structures and enhancing synergies between research infrastructures to developing regulatory compliance frameworks and engaging with industry, EVORA's WPs cover a spectrum of activities aimed at fortifying Europe's capacity to combat infectious diseases. Explore further to discover how each WP contributes to our mission of safeguarding public health and securing our future against pandemics.
These work packages oversee the day-to-day management and coordination of the EVORA project. Tasks include ensuring compliance with EC regulations, coordinating activities among consortium members, managing financial administration, and developing a comprehensive Data Management Plan. In summary, WP1 & WP2 establish the framework for efficient project execution and coordination.
These work packages aim to boost EVORA's visibility and impact. Activities include crafting communication plans, creating materials, managing online presence, and engaging with stakeholders. Dissemination efforts span conferences, publications, webinars, and industry outreach. Results will be shared widely and stored in an online repository for accessibility.
Focuses on securing its sustainability and long-term impact. Objectives include solidifying EVA's position, forming a collaboration agreement, and engaging with decision-makers. Key tasks involve establishing legal entities, drafting collaboration agreements, and communicating with stakeholders to ensure ongoing support and awareness. Through strategic planning and engagement, EVORA aims to pave the way for sustained success in pandemic preparedness and response.
Plays a central role in EVORA by preparing coordinated support for users during inter-crisis and viral outbreak conditions. Tasks include establishing operational modes, developing administrative frameworks, and testing EVORA's responsiveness. Key actions involve defining governance structures, designing access modalities, and drafting legal frameworks. Through simulation exercises and evaluation, WP6 aims to ensure EVORA's readiness to effectively respond to viral outbreaks and provide seamless support to users.
Aims to establish a common language and facilitate access to data and resources for research in virology. Tasks include creating a project ontology for describing virological material and services, developing web applications based on this ontology, and providing guidelines for data management in the virology community. Additionally, it involves maintaining data providers and workflows for data deposition, as well as creating web applications to facilitate user access to EVORA's resources.
Focuses on homogenizing and improving the service offer through shared quality management guidelines, ensuring compliance with quality standards for biobanking and experiments in high containment facilities. It involves defining QMS standards applicable to virus collections, data management, and service provision, as well as measuring implementation through audits, workshops, and self-assessments to optimize and ensure compliance with EVORA's quality guidelines.
Focuses on policy recommendations for pathogen preparedness, particularly addressing Nagoya Protocol compliance, transparency in digital viral resource management, and the intersection of the Convention on Biological Diversity (CBD) and WHO Pandemic Preparedness Accord. Tasks include engaging with authorities for Nagoya compliance, developing a roadmap for digital viral resources, and translating EVORA's science for EU and international policymakers. The aim is to ensure compliance, transparency, and harmonization in managing viral resources and data for global health preparedness.
Focuses on bridging the gap between Research Use Only (RUO) laboratory-developed diagnostic assays and In Vitro Diagnostics Regulation (IVD-R) requirements. Tasks include qualifying lab-developed tests, reviewing regulations, and testing the performance of in vitro diagnostics (IVDs) during epidemics or pandemics. The goal is to prepare and validate protocols for diagnostics qualification, harmonize legal and regulatory components, and develop guidance for performance testing of IVDs in outbreak situations. Ultimately, the aim is to adapt diagnostics for epidemic and pandemic preparedness activities, ensuring compliance with regulatory standards.
Focuses on ethical and security considerations in scientific research. It aims to establish criteria for selecting user projects involving animal use and dual-use pathogens, ensuring compliance with legal and ethical standards. The initiative addresses the responsible use of animals, analyzing ethical review processes and proposing specific requirements for project selection. Additionally, it tackles the handling of dual-use pathogens, examining national and international regulations and seeking solutions for cross-border exchange challenges. The outcomes will inform EVORA's shared procedures and contribute to future policy-making in these areas.
The work planned in EVORA will revolve around the three pillars of synergy, interoperability and regulation, in line with our objectives
Our partners bring together diverse yet complementary expertise, forming the foundation of EVORA's integrated approach to pandemic research and resource provision. EVORA, comprised of three partner Research Infrastructures (ELIXIR, ERINHA, EVA), leverages this collective expertise through collaboration and innovation. Together, they aim to strengthen Europe's capacity for pandemic preparedness and response, safeguarding global health and security.
ELIXIR specializes in data management and bioinformatics, providing essential support for organizing and analyzing large-scale life science data. Their expertise ensures efficient data sharing and collaboration, enhancing research outcomes in pandemic preparedness and response.
ERINHA is a key player in high containment research, offering
state-of-the-art
facilities and expertise for studying highly pathogenic agents. Their
focus on
biosafety and biosecurity ensures the safe handling of dangerous
pathogens,
contributing significantly to pandemic readiness.
EVA is a leading research infrastructure specializing in the collection, preservation, and distribution of viruses for scientific research. With extensive experience in virus resource provision, EVA plays a crucial role in facilitating access to viral materials essential for pandemic preparedness and response.
EVORA is built upon three partner Research Infrastructures (ELIXIR, ERINHA, EVA), each consisting of numerous partner institutions. Among these, the following institutions are actively engaged in the EVORA project . . .
The EVORA project delivers open, FAIR, and interoperable tools to support the virology community — including ontologies, APIs, data management guidelines, submission tools and repositories that strengthen how viral resources are shared and reused. Explore our public results and resources below.
Official results and deliverables of the EVORA project are published on the European Commission’s CORDIS platform.
View on CORDIS →Browse the EVORA project’s open-source repositories, featuring ontology development, APIs, and web showcase applications.
Visit EVORA on GitHub →Access open data, presentations, and materials deposited under the EVORA community on Zenodo.
Explore Zenodo Community →Developed in collaboration with ELIXIR, this new RDMkit Virology domain page provides guidelines for FAIR data management in virology, created through EVORA WP7.
Read the RDMkit Virology domain Page →
The EVORAO ontology (released by EVORA
under a CC0 license) unifies how viral materials and services are described across EVORA partners and
beyond, providing a structured vocabulary that enables semantic interoperability across the virology
community and related domains. Available on GitHub and resolvable through its w3id.org/evorao/
namespace in the Ontology Lookup Service (OLS).
The ICTV ontology developed by EVORA in collaboration with the International Committee on Taxonomy of Viruses (ICTV), transforms official virus taxonomy into FAIR, queryable data through the OLS API. The ICTV Resolver showcases how to use this new ICTV public API and related code helpers library to trace virus names and lineages.
This Galaxy-based workflow simplifies the submission of viral raw reads and genome assemblies to the European Nucleotide Archive (ENA), eliminating the need for command-line skills. It integrates the ENA Upload Tool and ENA Webin-CLI into a single, intuitive process that ensures metadata consistency and supports bulk submissions.
The tool is already available on the Belgian Galaxy instance, and will be progressively deployed on other major Galaxy platforms.
AMU UVE-UMR190
Emerging Viral Diseases
Aix-Marseille Université - IRD_190 - Inserm_1207
EFS - IRBA
Medical Faculty
27 boulevard Jean Moulin
13385
Marseille cedex 5 - France
Email us for any general queries, including colaboration opportunities.